Immunization Information: Vaccines, Schedules, and Benefits
Immunization ALSO KNOWN AS Vaccination is a critical component of preventative health care. It helps protect individuals and communities from dangerous and potentially life-threatening diseases. Immunization defines when a vaccine is administered to someone to help protect self an infectious disease. Vaccines work by stimulating the body’s natural defenses to create immunity to disease, which allows the body to combat and protect itself from illness. Immunization is a safe and effective way to help protect individuals from a wide range of diseases. Immunizations are available for infants, children, adolescents, adults, and seniors, and are important for everyone who is eligible. Please keep reading for details on the following topics:
Reasons why people get immunized
Vaccine
Chicken pox
Diphtheria. tetanus and pertussis
Hemophilus influenzae
Hepatitis A
Hepatitis B
Measles, mumps and rubella
Pneumococcal
Polio
Rotavirus
Meningococcal
Pneumococcal
Flu
Huma papillomavirus
Shingles
Precautions with vaccines
Immunizations and pregnancy
When to seek urgent medical care
Vaccines are essential tools in preventing infectious diseases and safeguarding public health. They work by stimulating the body’s immune system to recognize and fight off specific pathogens, such as bacteria or viruses. By introducing a harmless form of the pathogen or its components into the body, vaccines prompt the immune system to produce antibodies, which provide immunity against future infections.
Types of Vaccines:
- Live Attenuated Vaccines: These vaccines contain weakened forms of the virus or bacteria, stimulating a strong immune response without causing illness. Examples include measles, mumps, and rubella (MMR) vaccine.
- Inactivated Vaccines: These vaccines contain killed pathogens, which cannot cause disease but still stimulate an immune response. Examples include the polio vaccine.
- Subunit, Recombinant, Polysaccharide, and Conjugate Vaccines: These vaccines use specific components of the pathogen to trigger an immune response. Examples include the hepatitis B vaccine.
- mRNA Vaccines: These vaccines introduce a small piece of the virus’s genetic material into the body, instructing cells to produce a harmless protein that triggers an immune response. Examples include the COVID-19 mRNA vaccines.
Benefits of Vaccines:
- Disease Prevention: Vaccines prevent illnesses that can lead to serious health complications, hospitalization, and even death.
- Herd Immunity: Vaccination of a significant portion of the population reduces the spread of diseases, protecting vulnerable individuals who cannot be vaccinated.
- Eradication of Diseases: Vaccination efforts have led to the eradication of diseases such as smallpox and near-elimination of polio.
- Cost-Effectiveness: Vaccines are a cost-effective public health intervention, saving healthcare costs associated with treating preventable diseases.
Importance of Vaccines:
- Protecting Public Health: Vaccines are essential for maintaining public health and preventing outbreaks of infectious diseases.
- Ensuring Individual Health: Vaccination protects individuals from serious illnesses, improving overall health and well-being.
- Supporting Community Immunity: High vaccination rates within communities create herd immunity, reducing the spread of diseases and protecting vulnerable populations.
- Global Health Security: Vaccination efforts contribute to global health security by preventing the spread of infectious diseases across borders.
REASONS WHY PERSONS RECEIVE IMMUNIZATION
-
Protection against communicable diseases: Vaccines are designed to stimulate the body’s immune response to specific pathogens, thereby preventing infection and reducing the risk of developing serious illnesses. By receiving vaccinations, individuals can build immunity against various communicable diseases, reducing their susceptibility to infection and its associated complications.
- Prevention of disease transmission: Immunization not only protects vaccinated individuals but also helps prevent the spread of infectious diseases within communities. By achieving herd immunity, where a large proportion of the population is immunized against a particular disease, the overall transmission of the pathogen is significantly reduced. This helps protect vulnerable individuals who may be unable to receive vaccinations due to medical reasons and contributes to public health by limiting the spread of contagious diseases.
- Facilitation of international travel: Many countries require proof of vaccination against certain diseases as a prerequisite for entry, particularly for travelers coming from regions where those diseases are prevalent. Immunization ensures that travelers are protected against infectious diseases commonly encountered in foreign countries and helps prevent the importation and spread of diseases across borders. Additionally, some vaccinations may be recommended or required based on the specific health risks associated with travel to certain regions or countries.
- Workplace safety: In environments where employees may be exposed to infectious agents, such as healthcare facilities, laboratories, or food service establishments, immunization helps protect both employees and clients or customers from the transmission of diseases. For example, healthcare workers are often required to receive vaccinations against diseases like influenza, hepatitis B, and measles to minimize the risk of infection and ensure the safety of patients and colleagues.
- Legal and regulatory requirements: Some industries or employers may have legal or regulatory mandates regarding immunization for certain diseases. Compliance with these requirements is essential for maintaining accreditation, licensing, or certification standards and avoiding legal liability. Failure to adhere to immunization requirements may result in disciplinary action or limitations on employment opportunities.
- Protection of vulnerable populations: In settings where employees interact with individuals who may be more susceptible to severe complications from infectious diseases, such as children, elderly individuals, or immunocompromised individuals, immunization is crucial for preventing disease transmission and safeguarding the health of vulnerable populations.
SIDE EFFECTS OF VACCINES
These are potential side effects that may occur after receiving immunizations:
- Pain and swelling at the injection site: It is common to experience mild pain, redness, or swelling at the site where the vaccine was administered. This usually resolves within a few days and can be alleviated with over-the-counter pain relievers and cold compresses.
- Fever: A low-grade fever may develop after immunization as the body’s immune system responds to the vaccine. Fever is typically mild and short-lived, but it’s important to stay hydrated and monitor temperature. Contact a healthcare provider if the fever persists or becomes high-grade.
- Drowsiness: Feeling tired or drowsy is a common side effect of some vaccines. It’s advisable to rest and avoid strenuous activities after immunization, especially if feeling fatigued.
- Rash: Some individuals may develop a rash after receiving certain vaccines. Rashes are usually mild and resolve on their own without treatment. However, if the rash is severe or accompanied by other symptoms, it’s recommended to consult a healthcare professional.
- Joint pain: Joint pain or muscle aches can occur as a side effect of vaccination. This is usually mild and temporary, but individuals can alleviate discomfort by resting, applying heat or cold packs, and taking over-the-counter pain relievers as directed.
- Trouble breathing: While rare, some individuals may experience difficulty breathing or shortness of breath after vaccination. If experiencing severe respiratory symptoms such as wheezing or chest tightness, seek immediate medical attention.
-
Rapid heartbeat: In some cases, individuals may notice an increase in heart rate or palpitations after immunization. This is usually temporary and not cause for concern. However, if rapid heartbeat persists or is accompanied by other symptoms, it’s advisable to seek medical evaluation.
DISEASES WHICH PERSONS IMMUNIZATION AGAINST:
Bacterial meningitis
Diphtheria, tetanus, and pertussis (also known as whooping cough)
Flu (influenza)
Hemophilus influenzae type b disease, or Hib disease
Hepatitis A
Human papillomavirus (HPV)
Measles, mumps, and rubella
Pneumococcal disease
Polio
Rotavirus
Chickenpox (varicella) MMR
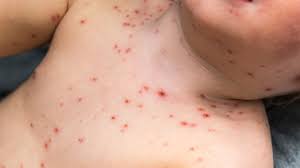
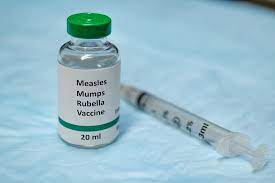
Chickenpox (varicella) is a common childhood illness caused by the varicella-zoster virus. The virus is highly contagious and can spread quickly among children. Symptoms include an itchy rash with blisters, fever, and fatigue. The best way to protect against chickenpox is to get the MMR vaccine, which is a combination of three vaccines that protect against measles, mumps, and rubella. The MMR vaccine is safe and effective in preventing chickenpox, as well as other serious illnesses.
The vaccine for measles, mumps, and rubella (MMR) is administered as follows:
- Timing of doses:
- The first dose is typically given between 12 to 15 months of age.
- A second dose is administered at 4 to 6 years of age, before starting school.
- Booster for unvaccinated individuals:
- Individuals aged 13 years or older who were not vaccinated during childhood should receive two separate doses of the MMR vaccine, with the doses given several weeks apart. This helps ensure adequate protection against measles, mumps, and rubella.
-
Exclusion for pregnant women:
- The MMR vaccine should not be administered to pregnant women due to potential risks to the developing fetus.
- However, it can be safely administered to women after giving birth, during the postpartum period.
Diphtheria, tetanus, and pertussis (DTaP)
The Diphtheria, Tetanus, and Pertussis (DTaP) vaccine is a safe and effective vaccine that provides long-term protection against three serious bacterial infections:
- Diphtheria: A bacterial infection that affects the throat and upper respiratory tract. It can cause breathing problems, paralysis, heart failure and even death.
- Tetanus: Also known as lockjaw, tetanus is caused by a toxin produced by the bacterium Clostridium tetani. It can cause severe muscle spasms, particularly in the jaw and neck muscles, seizures and death.
- Pertussis (Whooping Cough): A highly contagious respiratory infection characterized by severe coughing fits. Pertussis can be particularly dangerous for infants, leading to complications such as pneumonia, seizures, difficulty breathing and even death.

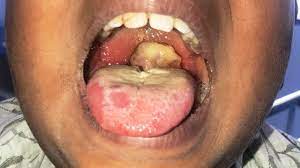
The DTaP vaccination schedule and additional information are as follows:
-
Vaccination Schedule:
- Infants typically receive DTaP doses at 2 months, 4 months, and 6 months of age as part of routine childhood immunization.
- A fourth dose is administered between 15 and 18 months of age to ensure continued protection.
- A fifth dose is given at 4-6 years old, usually before a child starts school.
- Lastly, a booster dose is recommended at 11-12 years of age to provide additional immunity during adolescence.
- Adult Immunity:
- While the vaccine schedule primarily focuses on childhood immunization, adults can maintain immunity by receiving booster doses every 10 years. This ensures ongoing protection against diphtheria, tetanus, and pertussis.
-
Pregnant Women:
- Pregnant women can receive a DTaP vaccination during the third trimester, between 27 and 36 weeks of gestation. This helps provide protection to both the pregnant woman and her newborn against pertussis, reducing the risk of transmission to the infant.
Hemophilus influenzae type b (Hib)
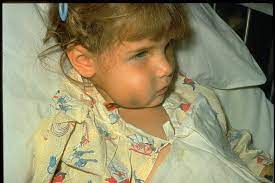
Hemophilus influenzae type b, or Hib, is an infectious disease caused by the bacterium of the same name. It is most common in young children but can affect individuals of any age. Symptoms can vary, ranging from mild cold-like symptoms to severe swelling around the brain and spinal cord, pneumonia, meningitis, bloodstream infections and other life-threatening illnesses particularly in infants and young children. Hemophilus influenzae type b (Hib) vaccine is essential for protecting against infections caused by the bacterium Haemophilus influenzae type b.
The Hib vaccine is crucial for protecting against Hib-related diseases, including:
- Meningitis: Hib meningitis is an infection of the membranes covering the brain and spinal cord. It can lead to serious complications, including brain damage and hearing loss.
- Pneumonia: Hib pneumonia is a lung infection that can cause difficulty breathing, coughing, and fever. Severe cases may result in respiratory failure.
- Epiglottitis: This is a life-threatening condition where the epiglottis, a tissue in the throat, becomes swollen and obstructs the airway, leading to breathing difficulties.
-
Septicemia: Also known as blood poisoning, septicemia occurs when the bacteria enter the bloodstream, causing a widespread infection that can lead to organ failure and death.
Vaccination Schedule:
- The Hib vaccine is typically administered in a series of doses to infants and young children to provide immunity against Hib infections.
- The standard immunization schedule includes doses at 2, 4, and 6 months of age, followed by a booster dose between 12 and 15 months of age.
Additional Doses:
- Some vaccine formulations may require a booster dose between 12 and 18 months of age to ensure long-term immunity.
- In some cases, catch-up doses may be recommended for older children who missed their initial vaccinations.
Hepatitis A (Hep A)
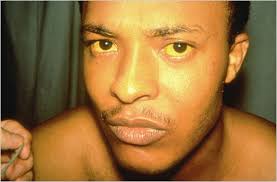
Hepatitis A, often referred to as Hep A, is a contagious virus that affects the liver. The virus is usually spread through contact with an infected person’s feces, contaminated food or water, or objects and surfaces that have come into contact with the virus. Hepatitis A vaccine can cause a range of symptoms, including:
- Jaundice: Yellowing of the skin and eyes due to liver dysfunction.
- Fatigue: Extreme tiredness and weakness.
- Nausea and vomiting: Digestive symptoms that can lead to dehydration.
- Abdominal pain: Discomfort or pain in the upper right side of the abdomen.
- Fever: Elevated body temperature.
- Loss of appetite: Reduced desire to eat.
- Dark urine: Urine may appear dark or tea-colored.
- Joint pain: Aches and pains in the joints.
Hepatitis A is typically spread through the ingestion of contaminated food or water or close contact with an infected person. The good news is that most people who contract Hep A can recover fully with no long-term effects. The Hepatitis A vaccine is highly effective in preventing infection and is recommended for:
- Travelers: People traveling to regions with high rates of Hepatitis A are advised to receive the vaccine before departure.
- High-risk individuals: Those with chronic liver disease, men who have sex with men, and people who use illicit drugs are at increased risk and may benefit from vaccination.
- Outbreak situations: During outbreaks or in communities experiencing an increased incidence of Hepatitis A, vaccination may be recommended to prevent further spread.
Vaccination Schedule:
The Hepatitis A vaccine is typically administered in two doses, with the second dose given 6 to 12 months after the first dose. The vaccine is usually administered as an injection into the muscle (intramuscular injection). The first dose provides initial protection, while the second dose ensures long-term immunity. Immunization is recommended for:
- Children starting at 12-24 months of age
- Adults who are at increased risk of infection or complications
- Individuals traveling to regions where Hepatitis A is endemic
- Certain high-risk groups, including healthcare workers and people with chronic liver disease
Hepatitis B (Hep B) is a viral infection that affects the liver. It is caused by the hepatitis B virus (HBV) and is spread through contact with the blood or other body fluids of an infected person. It can be spread through needle sharing, sexual contact, and from mother to baby during childbirth. Symptoms of Hep B can range from mild to severe, and while most people recover after a few weeks, some people can develop chronic Hep B. Those infected with chronic Hep B are at risk of developing complications such as cirrhosis and liver cancer. There are vaccines available to protect against the virus, and treatments are available for those living with chronic Hep B.
Vaccination Schedule:
The Hepatitis B vaccine is usually given as an injection into the muscle (intramuscular injection). It is recommended for:
- First Dose: Given shortly after birth, typically within 24 hours for newborns.
- Second Dose: Administered approximately 1 to 2 months after the first dose.
- Third Dose: Given approximately 6 to 18 months after the first dose.
- Children and adolescents who were not vaccinated as infants.
- Adults at increased risk of Hepatitis B infection, including healthcare workers, people with multiple sexual partners, people who inject drugs, when travelling and individuals with certain medical conditions.
Measles, mumps, and rubella (MMR)
The Measles, Mumps, and Rubella (MMR) vaccine is a combination vaccine that provides protection against three contagious viral infections: measles, mumps, and rubella. Here’s an overview of MMR:
- Measles: Measles is a highly contagious viral infection characterized by a distinctive rash. It spreads through respiratory droplets and can cause serious complications such as pneumonia, encephalitis (swelling of the brain), and death.
- Mumps: Mumps is caused by a virus spread through saliva and can lead to painful swelling in the salivary glands, as well as complications such as meningitis, deafness, and orchitis (swelling of the testicles).
-
Rubella: Rubella, also known as German measles, is a viral infection that causes a mild rash and fever. However, if contracted by pregnant women, rubella can lead to serious birth defects and miscarriage.
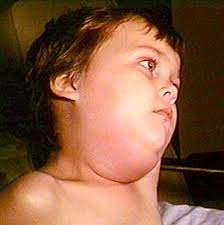
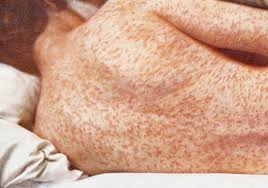
Vaccination Schedule:
The MMR vaccine contains weakened or inactivated forms of the viruses, which stimulate the immune system to produce protective antibodies. It is recommended for all children over the age of 12 months. The vaccine is typically administered in two doses:
-
- First Dose: Given to children between 12 and 15 months of age.
- Second Dose: Administered between 4 and 6 years of age, before starting school.
- A booster dose may be required during community outbreaks
Common side effects may include mild fever, rash, and temporary soreness or swelling at the injection site. Serious side effects are rare.
Pregnancy: The MMR vaccine should not be administered to pregnant women, as it contains live viruses that could potentially harm the fetus. Women should avoid becoming pregnant for at least one month after receiving the MMR vaccine.
Pneumococcal (PCV13)
Pneumococcal (PCV13) is a vaccine designed to protect against 13 of the most dangerous types of caused by the bacteria Streptococcus pneumoniae which can cause serious illnesses such as pneumonia, meningitis, bacteremia (bloodstream infection), and otitis media (middle ear infection). These infections can be severe, especially in young children, older adults, and individuals with weakened immune systems. It is recommended for children over the age of six weeks, as well as adults who may be at greater risk of developing pneumococcal infections. By getting vaccinated with PCV13, it helps protect against the most common and serious forms of pneumococcal disease. The vaccine works by helping your body create antibodies that are specific to the bacteria in the vaccine. These antibodies can fight off the bacteria if it is encountered in the future, helping to protect you from illness.
Vaccination Schedule
-
Vaccine Type: PCV13 is a conjugate vaccine, meaning it contains pieces of the outer coating of the bacteria (polysaccharides) linked to a carrier protein. This design helps stimulate a stronger immune response, leading to better protection against pneumococcal infections.
- Recommended Age Groups: PCV13 is recommended for infants and young children, as well as certain high-risk adults. The vaccination schedule may vary depending on age and risk factors.
- The typical vaccination schedule for PCV13 in children includes:
- Primary Series: A series of doses given at 2 months, 4 months, 6 months, and a booster dose between 12 and 15 months of age.
- Catch-Up Doses: For children who missed doses or started the series late, catch-up doses may be recommended.
- The schedule for adults may include one or more doses depending on age, health status, and previous vaccination history.
- Side Effects: Like all vaccines, PCV13 may cause mild side effects such as pain or redness at the injection site, mild fever, irritability, or fussiness. Serious side effects are rare.
Polio
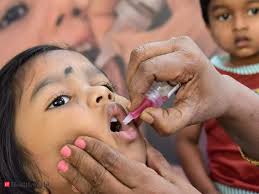
Polio, or poliomyelitis, is a highly contagious viral infection caused by the poliovirus. It primarily affects children under the age of 5. It can lead to paralysis, typically of the legs, and in severe cases, it can be life-threatening if the paralysis affects the muscles used for breathing. While most people infected with poliovirus do not develop symptoms, some may experience flu-like symptoms such as fever, headache, sore throat, fatigue, nausea, and stiffness in the neck and back.
Transmission: Poliovirus is spread primarily through person-to-person contact, typically through fecal-oral transmission or, less commonly, through respiratory droplets. This means that the virus spreads when an infected person’s stool or saliva contaminates food, water, or surfaces that are then ingested by another person.
Vaccination Schedule:
The polio vaccination schedule may vary depending on the country’s immunization program and the type of vaccine used. In general, children receive multiple doses of polio vaccine starting at 2 months of age, with booster doses administered at regular intervals to maintain immunity. There are two types of polio vaccines:
-
- Inactivated Poliovirus Vaccine (IPV): IPV is an injectable vaccine that contains killed poliovirus. It is typically administered as a series of doses during infancy and childhood.
- Oral Poliovirus Vaccine (OPV): OPV is a live attenuated vaccine that contains weakened poliovirus. It is administered orally and is often used in mass immunization campaigns due to its ease of administration and ability to provide community immunity.
Infants and Children: Four doses of the polio vaccine are typically administered to infants and children according to the following schedule:
- First dose: At 2 months of age
- Second dose: At 4 months of age
- Third dose: At 6-18 months of age
- Fourth dose: At 4-6 years of age
High-Risk Adults:
- For high-risk adults, three doses of the polio vaccine may be recommended. These individuals may include healthcare workers, laboratory personnel, travelers to areas where polio is endemic, and those with certain medical conditions that increase the risk of exposure to poliovirus.
Rotavirus

Rotavirus is a highly contagious virus that can cause severe diarrhea, vomiting, fever, and abdominal pain in young children. It is the leading cause of severe diarrhea among infants and young children worldwide and is responsible for over 200,000 deaths annually. The virus is easily spread from person to person through contact with an infected person’s stool, which can happen through contaminated food, water, or surfaces. The good news is that there are vaccines available that can help protect children from this virus. By vaccinating children, parents can help protect their children from the severe symptoms that Rotavirus can cause.
Vaccination Schedule:
Infants:
-
- Two doses of the rotavirus vaccine are recommended for infants to provide protection against rotavirus infection.
- The vaccine is usually administered orally, rather than by injection.
- The rotavirus vaccine is typically administered to infants in two doses, with the first dose given between 2-6 months of age and the second dose given before 8 months of age.
- The exact timing of doses may vary depending on the specific vaccine used and local immunization guidelines.
Side Effects:
-
- Common side effects of the rotavirus vaccine may include mild irritability, fussiness, or diarrhea.
- Serious side effects are rare but can occur, including allergic reactions.
Precautions:
-
- The rotavirus vaccine should not be administered to infants with a history of severe allergic reactions to any component of the vaccine.
- Healthcare providers should be informed of any allergies or underlying health conditions before administering the vaccine.
Meningococcal ACWY
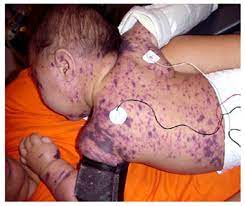
Meningococcal ACWY is an important vaccine that is recommended for everyone aged 11 to 18 years old. It helps to protect against four different strains of meningococcal bacteria, which can cause meningitis and septicemia. Meningococcal outbreaks can occur in community settings, particularly for adolescents and young adults who may be at increased risk due to social factors (e.g., college dormitory living) or medical conditions. Meningococcal ACWY is a safe, effective and long-lasting vaccine, with a single injection providing protection for at least five years. It can help to reduce the spread of this potentially fatal disease and help to protect those who have not had the vaccine from infection.
Pneumococcal (PPSV23)
Pneumococcal (PPSV23) is an important vaccine that can help protect people from a wide range of bacterial infections. It is recommended for adults over the age of 65, and those with certain underlying health conditions, including chronic conditions such as diabetes, heart or lung disease, and HIV/AIDS. The vaccine helps protect against 23 different types of pneumococcal bacteria, which can cause serious illnesses like pneumonia, sepsis, and meningitis. Although it may not prevent someone from contracting pneumonia, it can prevent severe complications attributed to the disease. It is recommended that adults get vaccinated at least once every 5 years to ensure their protection.
- Protection Against Pneumococcal Infections:
- PPSV23 vaccine helps protect against infections caused by the bacterium Streptococcus pneumoniae, commonly known as pneumococcus.
- Pneumococcal infections can lead to serious illnesses such as pneumonia, meningitis, and bloodstream infections.
- Vaccine Composition:
- PPSV23 contains purified polysaccharide antigens from 23 different strains of Streptococcus pneumoniae.
- The vaccine is designed to stimulate the body’s immune response to produce antibodies against these pneumococcal strains.
- Vaccine Schedule:
- The typical schedule for PPSV23 immunization involves a single dose for most adults aged 65 years and older.
- Individuals aged 19-64 years with certain medical conditions, such as diabetes, chronic heart, liver, or lung disease, as well as immunocompromised individuals, may also receive the vaccine.
- In some cases, a second dose may be recommended for individuals at continued high risk of pneumococcal disease, such as those with certain immune deficiencies.
- Side Effects:
- Common side effects of PPSV23 vaccine may include mild pain, redness, or swelling at the injection site, as well as mild fever or fatigue.
- Serious side effects are rare but may include severe allergic reactions or Guillain-Barré syndrome.
- Precautions:
- PPSV23 vaccine should not be administered to individuals with a history of severe allergic reactions to any component of the vaccine.
- Healthcare providers should be informed of any allergies or underlying health conditions before administering the vaccine.

Flu, also known as influenza, is a highly contagious respiratory illness caused by a virus. Symptoms of the flu can range from mild to severe and can include fever, chills, body aches, a sore throat, coughing, and fatigue. The flu can be prevented by getting a flu shot each year, washing your hands often, and avoiding contact with people who are sick. With prompt diagnosis and treatment, the majority of people can make a full recovery from the flu.
Vaccine Composition:
-
- Each year, the flu vaccine is updated to target the specific strains of influenza virus expected to circulate during the upcoming flu season.
- The vaccine may be formulated as a trivalent vaccine, targeting three different influenza strains, or a quadrivalent vaccine, targeting four strains.
- Flu vaccines may be made using inactivated (killed) virus, live attenuated (weakened) virus, or recombinant technology.
Vaccine Administration:
-
- The flu vaccine is typically administered as an injection into the muscle (intramuscular) of the upper arm or thigh.
- Some flu vaccines are also available in the form of a nasal spray, which is administered into the nostrils.
Vaccine Schedule:
-
- Annual vaccination is recommended before the start of each flu season, typically in the fall or early winter.
- Immunity from the flu vaccine wanes over time, so annual vaccination is necessary to ensure continued protection.
- Vaccination is particularly important for high-risk individuals, healthcare workers, and those in close contact with vulnerable populations.
Side Effects:
-
- Common side effects of the flu vaccine may include mild soreness, redness, or swelling at the injection site, as well as low-grade fever, headache, or muscle aches.
- Serious side effects are rare but may include severe allergic reactions.
Precautions:
-
- The flu vaccine should not be administered to individuals with a history of severe allergic reactions to any component of the vaccine.
- Individuals with certain medical conditions, such as severe egg allergy or Guillain-Barré syndrome, should consult with their healthcare provider before receiving the flu vaccine.
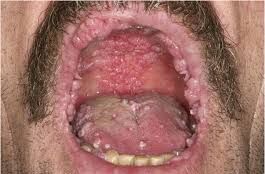
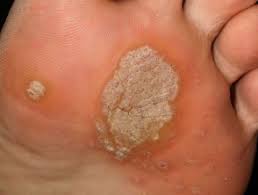
Human papillomavirus (HPV) is a common virus that affects most people at some point in their lives. It is spread through skin-to-skin contact and can cause genital warts, as well as other types of cancer. Although there is no cure for HPV, it can be managed with regular screenings and treatments. Vaccines are also available to help protect against the virus. With proper care, people can reduce their risk of developing HPV-related health problems.
Vaccine Composition:
-
- The HPV vaccine is typically composed of virus-like particles (VLPs) that mimic the structure of the HPV virus but do not contain the viral DNA, making them unable to cause infection.
- Depending on the formulation, the vaccine may protect against two, four, or nine HPV types.
Vaccine Administration:
-
- The HPV vaccine is administered as a series of injections into the muscle (intramuscular) of the upper arm or thigh.
- The number of doses and the schedule may vary depending on the age at initial vaccination and the specific HPV vaccine product used.
Vaccine Schedule:
-
- The HPV vaccine is typically administered as a two-dose series for individuals aged 9 to 14 years, with the doses given six to twelve months apart.
- For individuals aged 15 to 26 years, a three-dose series is usually recommended, with the second dose given 1-2 months after the first dose, and the third dose given 6 months after the first dose.
Side Effects:
-
- Common side effects of the HPV vaccine may include mild soreness, redness, or swelling at the injection site, as well as headache, fatigue, or fever.
- Serious side effects are rare but may include severe allergic reactions.
Precautions:
-
- The HPV vaccine is generally safe and well-tolerated, but it should not be administered to individuals with a history of severe allergic reactions to any component of the vaccine.
- Individuals who are pregnant should delay vaccination until after the pregnancy.
- Routine Pap screening and HPV testing are still recommended for women, even if they have been vaccinated against HPV.
Shingles (herpes zoster)
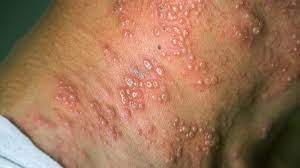
Shingles, also known as herpes zoster, is a painful, contagious rash caused by the varicella-zoster virus, the same virus that causes chickenpox. It typically appears as a band or a strip of raised dots on one side of the body or face and can cause intense itching, burning, and pain. In some cases, shingles can cause long-term nerve damage, called postherpetic neuralgia. Treatment for shingles typically involves antiviral medications, pain relief medications, and home remedies.
2 doses are given to adults at 50 years 2 – 6 months apart and one dose to adults above 60 years.
Vaccine Composition:
-
- The shingles vaccine is typically composed of a weakened form of the varicella-zoster virus (live attenuated virus) that stimulates the immune system to produce antibodies against the virus.
- The vaccine is a combination of 2 products Shingrix (RZV) and Zostavax (ZVL)
- The vaccine helps the immune system recognize and respond to the varicella-zoster virus more effectively, reducing the likelihood of shingles development.
Vaccine Administration:
-
- The shingles vaccine is administered as a single dose injection under the skin (subcutaneous) of the upper arm.
- It is important to receive the vaccine even if you have previously had shingles, as it can help prevent future episodes or reduce their severity.
Vaccine Schedule:
-
- The shingles vaccine is typically recommended for adults aged 50 years and older, regardless of whether they have had chickenpox or shingles in the past.
- Some countries may have specific guidelines regarding the age at which vaccination is recommended, so it is essential to consult with a healthcare provider.
Side Effects:
-
- Common side effects of the shingles vaccine may include mild soreness, redness, or swelling at the injection site.
- Some individuals may experience headache, fatigue, or low-grade fever following vaccination, but these symptoms are usually mild and temporary.
Precautions:
-
- The shingles vaccine is generally safe for most individuals, but it should not be given to individuals who have a severe allergic reaction to any component of the vaccine.
- Pregnant women and individuals with weakened immune systems, such as those undergoing cancer treatment or taking immunosuppressive medications, should not receive the shingles vaccine.
PRECAUTIONS WITH VACCINES
-
Monitoring for Adverse Reactions:
- Individuals receiving vaccines should be monitored for at least 15 minutes after vaccination for any signs of adverse reactions.
- Healthcare providers should be prepared to address any immediate reactions that may occur, such as allergic reactions or fainting.
- Healthcare providers and individuals should report any suspected adverse events following vaccination to the appropriate authorities, such as the Vaccine Adverse Event Reporting System (VAERS).
- Timely reporting of adverse events helps monitor vaccine safety and facilitates ongoing surveillance and evaluation of vaccine-related risks.
- Seeking Medical Advice:
- If individuals have any concerns or questions about vaccines, they should seek advice from a qualified healthcare professional.
- It is important to discuss any medical conditions, allergies, or previous adverse reactions with the healthcare provider before receiving vaccination.
- Storage of Immunization Records:
- Immunization records should be stored in a safe and accessible location.
- Keeping accurate records of vaccines received is essential for ensuring individuals are up-to-date with their vaccinations and for providing documentation when needed.
- Reporting Adverse Events:
- Individuals who experience adverse events following vaccination should report these events to their healthcare provider or the appropriate public health authorities.
- Reporting adverse events helps healthcare providers and regulatory agencies monitor vaccine safety and take appropriate actions if needed.
- Adherence to Vaccine Schedules:
- It is important to adhere to recommended vaccine schedules and receive all necessary doses according to the recommended intervals.
- Missing doses or delaying vaccination may reduce the effectiveness of vaccines and leave individuals susceptible to vaccine-preventable diseases.
- Informed Decision-Making:
- Individuals should make informed decisions about vaccination based on accurate information from reliable sources.
- Healthcare providers play a crucial role in providing information about the benefits and risks of vaccination, as well as addressing any concerns or misconceptions individuals may have.
- Special Considerations:
- Certain populations, such as pregnant women, individuals with weakened immune systems, and those with specific medical conditions, may have special considerations regarding vaccination.
- Healthcare providers should assess individual risk factors and provide personalized recommendations for vaccination based on current guidelines and evidence-based practices.
- Continued Surveillance and Research:
- Ongoing surveillance and research are essential for monitoring vaccine safety and effectiveness.
- Healthcare providers, researchers, and public health authorities collaborate to identify and address emerging issues related to vaccines, ensuring continuous improvement in vaccine development and administration practices.
- Allergic Reactions:
- Individuals with a history of severe allergic reactions (anaphylaxis) to vaccine components, such as latex, should not receive the vaccine.
- Healthcare providers should be informed of any known allergies or previous adverse reactions before administering vaccines.
- Storage and Handling:
- Vaccines should be stored and handled according to recommended guidelines to maintain their potency and effectiveness.
- Proper storage conditions, including temperature control and protection from light, are crucial to prevent vaccine degradation.
- Pregnancy Considerations:
- Some vaccines may be contraindicated during pregnancy due to potential risks to the developing fetus.
- Pregnant individuals should consult with their healthcare provider before receiving any vaccines to assess the benefits and risks.
- Post-Vaccination Care:
- After vaccination, individuals should be advised on how to manage common post-vaccination reactions, such as pain at the injection site or mild fever.
- Adequate hydration, rest, and over-the-counter pain relievers may be recommended to alleviate discomfort and promote recovery.
IMMUNIZATIONS AND PREGNANCY
-
Preconception Planning:
- Before planning pregnancy, individuals should review their immunization history and ensure they are up to date on recommended vaccines.
- Certain vaccines, such as those for influenza and tetanus, are safe and recommended for administration before or during pregnancy to protect both the mother and the unborn child.
- Vaccines During Pregnancy:
- Some vaccines are safe and recommended during pregnancy to protect both the pregnant individual and their baby.
- The influenza (flu) vaccine is routinely recommended for pregnant individuals during flu season to reduce the risk of flu-related complications for both mother and baby.
- The Tdap (tetanus, diphtheria, and pertussis) vaccine is recommended during each pregnancy, ideally between 27 and 36 weeks gestation, to provide passive immunity to the newborn against pertussis (whooping cough).
- Vaccine Safety:
- Extensive research supports the safety and effectiveness of certain vaccines during pregnancy.
- Vaccines recommended during pregnancy undergo rigorous safety evaluations to ensure they do not pose harm to the pregnant individual or the developing fetus.
- Live Vaccines:
- Live attenuated vaccines, such as the measles, mumps, and rubella (MMR) vaccine, are generally contraindicated during pregnancy due to theoretical concerns about fetal harm.
- Pregnant individuals should avoid receiving live vaccines, and if inadvertently administered, they should consult with their healthcare provider for guidance.
- Vaccination Timing:
- Some vaccines are ideally administered before pregnancy to ensure optimal protection for both the mother and the baby.
- Preconception vaccination allows sufficient time for the immune system to develop protective immunity before conception and reduces the risk of vaccine-preventable diseases during pregnancy.
- Risk-Benefit Assessment:
- The decision to administer vaccines during pregnancy involves a careful risk-benefit assessment.
- Healthcare providers consider factors such as the individual’s vaccination status, risk of exposure to vaccine-preventable diseases, potential vaccine-related risks, and the stage of pregnancy when determining vaccine recommendations.
- Informed Decision-Making:
- Pregnant individuals should engage in shared decision-making with their healthcare providers regarding vaccination during pregnancy.
- They should receive clear and accurate information about the benefits and potential risks of vaccination to make informed decisions that align with their preferences and health needs.
- Postpartum Immunization:
- Immunizations that are contraindicated during pregnancy can be administered in the postpartum period.
- Healthcare providers may recommend catch-up vaccinations for individuals who missed vaccines during pregnancy or need additional doses for optimal protection.
-
Continued Immunization:
- Vaccination remains important beyond pregnancy to maintain immunity and protect against vaccine-preventable diseases for both the individual and their family members.
- Postpartum individuals should continue to follow recommended vaccination schedules and receive any missed or overdue vaccines as appropriate.
THINGS TO DO AT HOME
Things to Do at Home After Vaccination:
- Monitor for Side Effects:
- Keep an eye out for common side effects such as pain, swelling, or redness at the injection site, fever, fatigue, or mild flu-like symptoms.
- Note any unusual or severe reactions and contact your healthcare provider if you have concerns.
- Manage Fever:
- If you experience fever after vaccination, you can take over-the-counter fever-reducing medications such as acetaminophen or ibuprofen as directed by your healthcare provider.
- Stay hydrated by drinking plenty of fluids and get adequate rest.
- Ease Discomfort:
- Apply a cool compress or ice pack to the injection site to reduce pain and swelling.
- Avoid strenuous activities or heavy lifting that may exacerbate discomfort.
- Monitor for Allergic Reactions:
- Watch for signs of allergic reactions such as difficulty breathing, swelling of the face or throat, hives, or rapid heartbeat.
- Seek immediate medical attention if you experience any severe allergic reactions.
- Comfort Measures:
- Rest and relaxation can help alleviate discomfort and promote healing.
- Engage in activities that provide comfort and distraction, such as reading, listening to music, or watching movies.
- Follow-Up Care:
- Attend any scheduled follow-up appointments with your healthcare provider to monitor your response to the vaccine and address any concerns.
- Keep track of your immunization records and update them accordingly.
- Stay Informed:
- Stay informed about vaccine-related updates, including booster doses or new vaccine recommendations.
- Seek credible information from reputable sources such as healthcare providers, government health agencies, or reputable medical websites.
- Maintain Hygiene:
- Practice good hand hygiene by washing your hands frequently with soap and water for at least 20 seconds.
- Cover your mouth and nose with a tissue or your elbow when coughing or sneezing to prevent the spread of germs.
- Healthy Lifestyle:
- Maintain a healthy lifestyle by eating a balanced diet, staying physically active, and getting enough sleep.
- Avoid smoking and limit alcohol consumption to support your overall health and immune function.
- Stay Connected:
- Stay connected with your healthcare provider and reach out if you have any questions or concerns about your health or vaccination status.
- Stay connected with friends and family for social support and to share experiences related to vaccination.
11. Swelling or redness around the injection site
- Place a cool compression to the area for about 10-20 minutes
- Prescribed pain medication
12. Restlessness and poor appetite
- Hold baby when necessary to provide comfort
- Ensure a cool and comfortable temperature
- Seek urgent medical care or advice when necessary
WHEN TO SEEK URGENT MEDICAL CARE
It is important to know when to seek urgent medical care, as delaying appropriate treatment can lead to further health complications. If you or your child experience any of the following symptoms, you should seek medical help immediately. If you have any doubts about whether you should seek medical care, it is always better to err on the side of caution and consult with your doctor. Seek urgent medical care if you or your child experience any of the following symptoms after receiving a vaccine:
Difficulty breathing
Heart racing
Swelling to the body or throat
Unconsciousness
Confusion
Seizure
Inflammation to the limb or injection site
High fever
Poor appetite
Disclaimer: The information provided in this content is for general informational purposes only. It is not intended as medical or healthcare advice, diagnosis, or treatment. Always seek the advice of a qualified healthcare professional with any questions you may have regarding a medical condition or healthcare decisions. Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization Immunization

